Summary
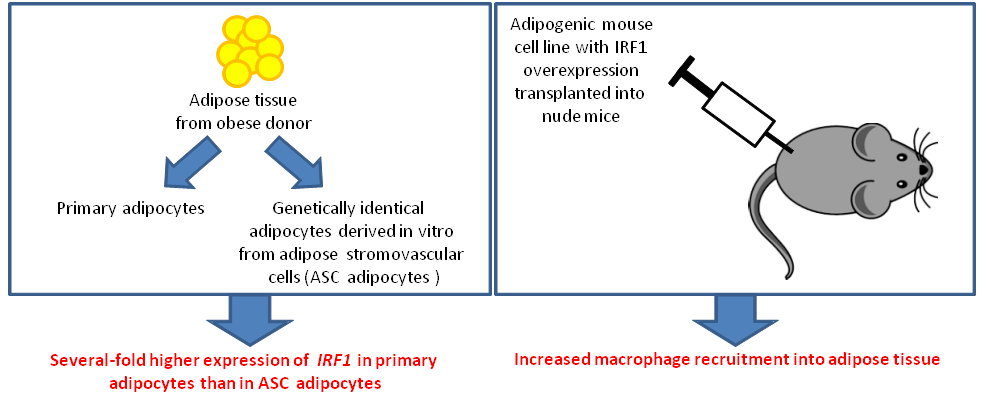
Chronic inflammation is thought to contribute to obesity-related insulin resistance. In obesity, adipocytes are an important source of inflammatory cytokines, but the mechanisms of adipose inflammation in obesity remain unclear. To determine transcriptional regulators of adipose inflammation, Cowan and colleagues compared transcriptional profiles of primary human adipocytes from obese donors with those from in vitro-derived adipocytes that were genetically identical to the primary adipocytes. Interferon regulatory factor 1 (IRF1) was identified as a mediator of adipocyte inflammation, as it was upregulated in primary adipocytes compared to genetically identical in vitro-derived adipocytes. Further investigation demonstrated that IRF1 overexpression in adipocytes resulted in upregulation of genes associated with innate immunity, with concomitant increases in the secretion of IL-6, IL-8, and monocyte chemotactic protein 1 (MCP1). IRF1 overexpression also led to decreased insulin sensitivity as well as reduced triglyceride hydrolysis in response to β-adrenergic stimulation. Together, these results demonstrate that IRF1 is a regulator of obesity-related inflammation and metabolic dysfunction.
Citation

